Researchers from the group of Prof. Gilles Gasser at the Institute of Chemistry for Life and Health Sciences at Chimie ParisTech–PSL, in collaboration with the groups of Prof. Gonçalo Bernardes (University of Cambridge) and Prof. Vicente Marchán (University of Barcelona), have developed a new metal-based molecule capable of delivering therapeutic amounts of carbon monoxide (CO) directly inside tumor cells. Although CO is best known as a toxic gas, when administered in small and controlled doses, it can have beneficial effects such as reducing inflammation, protecting tissues, and slowing cancer progression.
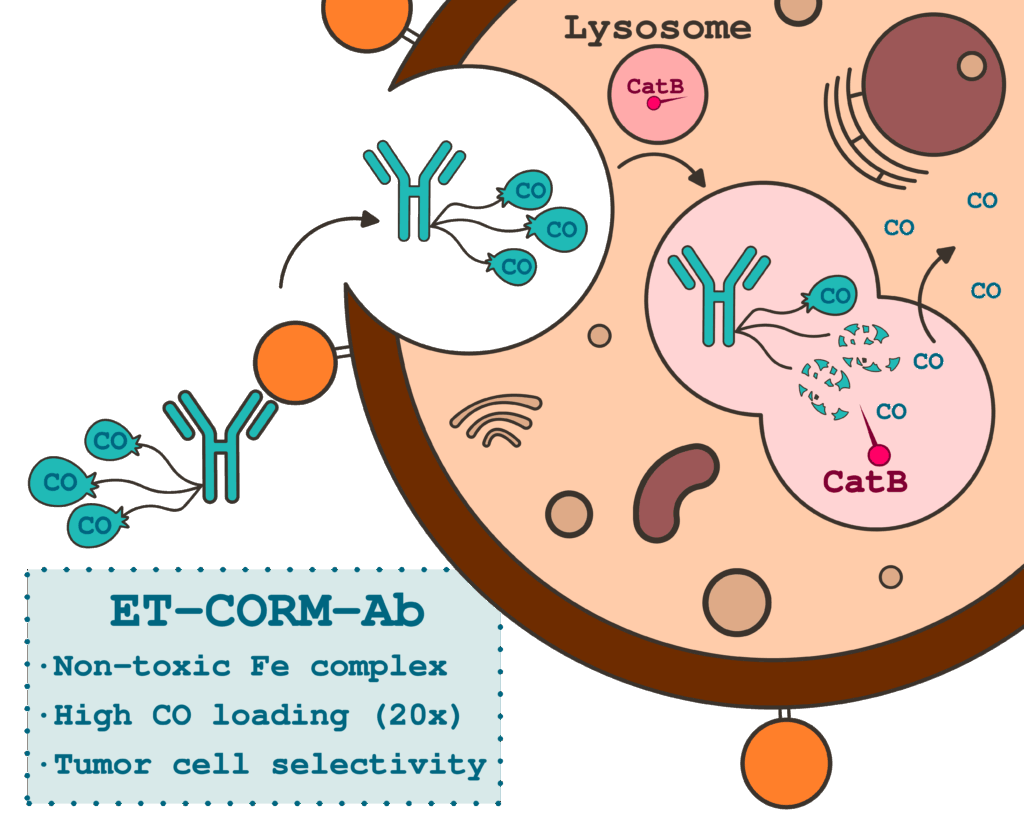
The team of scientists designed a carbon monoxide–releasing molecule (ET-CORM) built around a biocompatible iron core that showed no toxicity to cells. This molecule is specifically activated by cathepsin B, an enzyme abundantly expressed in many tumor cells. By conjugating ET-CORM to trastuzumab, a clinically approved antibody that targets HER2 receptors, biomarkers overexpressed in several cancers, the researchers created a targeted conjugate (ET-CORM-Ab) capable of releasing CO only in cells where both HER2 and cathepsin B are present. In laboratory studies, this conjugate successfully released CO within HER2-overexpressing breast cancer cells while leaving healthy or HER2-low cells unaffected.
This work represents the first antibody–metal conjugate that releases carbon monoxide in response to a tumor-specific enzymatic trigger. By combining the precision of antibody–drug conjugates with the emerging field of gas-based therapeutics, the study opens a safer and more selective route to harness the medical potential of carbon monoxide for the treatment of cancer and inflammatory diseases.
The study has been accepted for publication in Angewandte Chemie International Edition and is available online at https://doi.org/10.1002/anie.202513808.