Development of novel metal-based anticancer drug candidates
Chemotherapy is one of the leading anticancer strategies to cure cancer. Both the pharmaceutical industry and academia are highly devoted to the search of new chemotherapeutic agents. Platinum-based drugs are approved and used in the clinic against cancer. However, these treatments can lead to the occurrence of severe side effects, which are limiting their application. Ruthenium-based compounds are considered as the most promising second generation of anticancer metal-based drug candidates.1 Three Ruthenium complexes (i.e., KP-1019, KP-1339 and NAMI-A) have already entered clinical trials as chemotherapeutic agents against cancer.
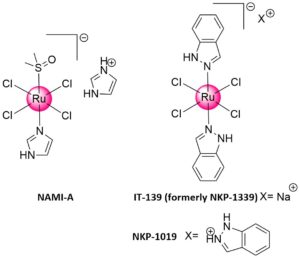
Figure 1. Chemical structures of Ruthenium complexes that were (i.e., NKP-1019 and NAMI-A, Figure 1) or are (i.e., IT-139) in clinical trials.
In our research group, novel Ru(II) complexes are synthesized, characterized and their biological activity investigated. Furthermore, the mechanism of action of these compounds is studied in depth using biochemical/molecular biological techniques Among these, the culture of Multicelluar Toumor Spheroids (MCTS, Figure 2), the study of cellular accumulation, intracellular distribution and DNA metalation via Inductively coupled plasma mass spectrometry (ICP-MS, Figure 3) or metabolic studies using Seahorse XF analyser (Figure 4).2–5

Figure 2. Changes in growth kinetics of Multicellular Tumour Spheroids (MCTS) treated with a cytotoxic complex at different concentrations (1, 5, 10, 20, 25 and 30 µM). (a) Images collected at day 0 (before treatment) and at day 3, 6, 9 and 13. b) MCTS diameter measured at different time points. Blue dotted line indicates day of seeding, red dashed line indicates day of treatment, green dotted lines indicate days of washing. Figure taken from ref. 2.

Figure 3. Cellular accumulation, intracellular distribution and DNA metalation analysed ICP-MS of two of our cytotoxic Ru(II) complexes. Figure taken from ref. 2.

Figure 4. Mito Stress Test profile after 24 h treatment; oxygen consumption rate changes after treatment with specific electron transport chain inhibitors. Figure taken from ref. 2.
More specifically, we could recently unveil a lead compound (Figure 2) through a structure activity relationship study. This compound exerted advantageous modes of action and an outstanding cytotoxicity. Moreover, despite its poor water solubility,6 this complex demonstrated to distribute well in vivo with the use of an appropriate formulation. Taken together these results makes our complex an interesting compound for clinical application in the search of potential chemotherapeutic agents against cancer.
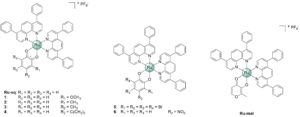
Figure 5. Structures of a cytotoxic Ru(II) complexes developed in our labs. Figure taken from ref. 2.
References
1. A. Notaro and G. Gasser, Chem. Soc. Rev., 2017, 46, 7317–7337.
2. A. Notaro, M. Jakubaszek, N. Rotthowe, F. Maschietto, R. Vinck, P. S. Felder, B. Goud, M. Tharaud, I. Ciofini, F. Bedioui, R. F. Winter and G. Gasser, J. Am. Chem. Soc., 2020, 142, 6066-6084.
3. A. Notaro, M. Jakubaszek, S. Koch, R. Rubbiani, O. Dömötör, É. A. Enyedy, M. Dotou, F. Bedioui, M. Tharaud, B. Goud, S. Ferrari, E. Alessio and G. Gasser, Chem. Eur. J., 2020, 26, 4997–5009.
4. A.-C. Munteanu, A. Notaro, M. Jakubaszek, J. Cowell, M. Tharaud, B. Goud, V. Uivarosi and G. Gasser, Inorg. Chem., 2020, 59, 4424-4434.
5. A. Notaro, A. Frei, R. Rubbiani, M. Jakubaszek, U. Basu, S. Koch, C. Mari, M. Dotou, O. Blacque, J. Gouyon, F. Bedioui, N. Rotthowe, R. F. Winter, B. Goud, S. Ferrari, M. Tharaud, M. Řezáčová, J. Humajová, P. Tomšík and G. Gasser, J. Med. Chem., 2020, DOI: 10.1021/acs.jmedchem.0c00431.
6. A. Notaro, G. Gasser and A. Castonguay, ChemMedChem, 2020, 15, 345-348.
