Development of novel Organometallic-based Antiparasitic Drug Candidates
Over the recent years, organometallic compounds have shown enormous potential in medicinal chemistry and chemical biology.1-4 An interesting concept in these fields has been the replacement of an organic part (e.g. phenyl ring) of an existing drug by an organometallic complex (e.g. ferrocene). This idea has been pioneered by the group of Gérard Jaouen at Chimie ParisTech by manipulating the organic anticancer drug Tamoxifen to produce the so-called Ferrocifens (Figure 1).2 The most successful example utilising this concept is undoubtedly the antimalarial drug candidate Ferroquine (Figure 1).3 Ferroquine is a ferrocenyl analogue of the antimalarial drug Chloroquine which is currently undergoing phase IIb clinical trial. For both Ferrocifen and Ferroquine, the addition of a metal complex has allowed metal-specific modes of action to be uncovered, which has enabled resistance to be overcome and/or the bioactivity of the organic drug to be enhanced.
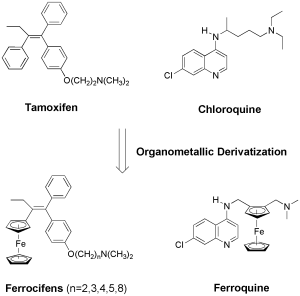
Figure 1. Structures of Tamoxifen, Ferrocifen, Chloroquine and Ferroquine.
In our group, we are currently using a similar concept to fight schistosomiasis. Schistosomiasis is the second most prevalent parasitic disease in the world after malaria. At the moment, schistosomiasis is treated with the organic drug Praziquantel (PZQ). However, PZQ has several drawbacks (i.e. low metabolic stability, inactivity against juvenile worms, etc.). But more worryingly, a decrease in activity has been observed in certain regions of the globe suggesting that PZQ could become (much less) effective in the future.5-7 Over the recent years, in collaborations with Prof. Jennifer Keiser from the Swiss Tropical and Public Health Institute in Basel, we have screened several organometallic compounds as novel antischistosomal drug candidates.8-14
One of the great interests of our group is to understand the stability in biological media and the metabolic path of our metal-based drug candidates.15 For example, we could recently perform lead optimization of validated drug candidates for delivery within an academic platform. We synthesised 3 lead compounds derived from Oxamniquine (OXA): ferrocene-containing (Fc-CH2-OXA), ruthenocene-containing (Rc-CH2-OXA) and benzene-containing (Ph-CH2-OXA).14 OXA is an old anthelmintic drug, which used to be mass-administered for the schistosomiasis eradication program in Brazil but has since been replaced by PZQ.8,16 These derivatives showed excellent in vitro activity against both S. mansoni and S. haematobium larvae and adult worms, and in vivo against S. mansoni. We followed up these compounds through metabolic stability studies,17 in vivo studies, computational simulations, and formulation studies. Of particular interest, the molecular dynamics simulations supported the in vitro results on the target protein, while the in vivo studies showed a lack of activity for all three derivatives against juvenile S. mansoni and adult S. haematobium (worm burden reduction 39 – 47 %). The the promising in vitro results in S. haematobium (IC50 15.5 – 52.3 mM) did not translate to in vivo activity (worm burden reduction 0 %) due to poor stability within an acidic environment and poor clearance in the in vitro liver model (intrinsic clearance 7.5 – 13.3 mL/min/mg). Lipid nanoencapsulation of Ph-CH2-OXA showed no improvement within S. mansoni in vivo studies (worm burden reduction 39 % before encapsulation, 0 % after encapsulation). Future strategies should include the study of gastro-resistant ways of drug administration, to ensure that improved bioavailability can overcome the loss of in vivo activity.
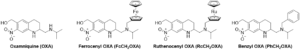
Figure 2. Structures of organometallic-containing OXA derivatives.
We are also working on helminthiases, a group of neglected tropical diseases, affect more than one billion people mainly in tropical and subtropical regions in collaboration with Prof. Andrew Hemphill at the University of Berne (Switzerland). Albendazole (ABZ) is a broad‐spectrum anthelmintic treatment recommended by the World Health Organization (WHO). However, drug resistance is emerging due to its widespread use. We report a series new ferrocenyl and ruthenocenyl ABZ derivatives (Figure 3) and assessed their activity against different helminths and protozoans, including Trichuris muris adults, Heligmosomoides polygygrus adults, Schistosoma mansoni adults, Giardia lamblia, Haemonchus contortus xL3s and Toxoplasma gondii. In particular, ferrocenyl containing 2d exhibited > 70% selective in vitro activity against T. muris adults up to 100 μM. Against T. gondii, ferrocenyl containing 1a and 2d showed improved activity compared to the parent ABZ. 1b and 2d showed moderate worm burden reductions and low worm explusion rates in T. muris infected mice with a 400 mg/kg dose. Testing these compounds on a broad range of parasitic helminths and protozoan confirms the potential of organometallic complexes against parasites of medical and veterinary importance.18
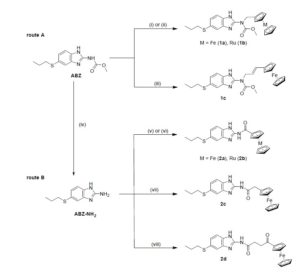
Figure 3. Structures of organometallic-containing ABZ derivatives.
References
- Gasser, G.; Metzler-Nolte, N. Curr. Opin. Chem. Biol. 2012, 16, 84.
- Hillard, E. A.; Vessières, A.; Jaouen, G. In Medicinal Organometallic Chemistry; Jaouen, G., Metzler-Nolte, N., Eds.; Springer-Verlag: Heidelberg, 2010; Vol. 32, p 81.
- Biot, C.; Dive, D. In Medicinal Organometallic Chemistry; Jaouen, G., Metzler-Nolte, N., Eds.; Springer-Verlag: Heidelberg, 2010; Vol. 32, p 155.
- Patra, M., Gasser , G. Nature Rev. Chem. 2017, 1, 0066.
- Ismail, M.; Botros, S.; Metwally, A.; William, S.; Farghally, A.; Tao, L. F.; Day, T. A.; Bennett, J. L. Am. J. Trop. Med. Hyg. 1999, 60, 932.
- Melman, S. D.; Steinauer, M. L.; Cunningham, C.; Kubatko, L. S.; Mwangi, I. N.; Wynn, N. B.; Mutuku, M. W.; Karanja, D. M. S.; Colley, D. G.; Black, C. L.; Secor, W. E.; Mkoji, G. M.; Loker, E. S. PLoS Negl. Trop. Dis. 2009, 3, e504.
- Greenberg, R. M. Parasitology 2013, 140, 1534.
- Ong, Y. C.; Roy, S.; Andrews, P. C.; Gasser, G. Chem. Rev. 2019, 119, 730.
- Hess, J.; Keiser, J.; Gasser , G. Future. Med. Chem. 2015, 8, 821.
- Patra, M.; Ingram, K.; Leonidova, A.; Pierroz, V.; Ferrari, S.; Robertson, M.; Todd, M. H.; Keiser, J.; Gasser, G. J. Med. Chem. 2013, 56, 9192.
- Patra, M.; Ingram, K.; Pierroz, V.; Ferrari, S.; Spingler, B.; Gasser, R. B.; Keiser, J.; Gasser, G. Chem. Eur. J. 2013, 19, 2232.
- Patra, M.; Ingram, K.; Pierroz, V.; Ferrari, S.; Spingler, B.; Keiser, J.; Gasser, G. J. Med. Chem. 2012, 55, 8790.
- d’Orchymont, F.; Hess, J.; Panic, G.; Jakubaszek, M.; Gemperle, L.; Keiser, J.; Gasser, G. Med. Chem. Commun. 2018, 9, 1905.
- Hess, J.; Panic, G.; Patra, M.; Mastrobuoni, L.; Blacque, O.; Roy, S.; Keiser, J.; Gasser, G. ACS Infect. Dis. 2017, 3, 645.
- Keller, S.; Ong, Y. C.; Lin, Y.; Cariou, K.; Gasser, G. J. Organomet. Chem. 2020, 906, 121059.
- Ong, Y. C.; Gasser, G. Discov. Today Technol. 2019, DOI: 16/j.ddtec.2019.06.001.
- Buchter, V.; Ong, Y. C.; Mouvet, F.; Ladaycia, A.; Lepeltier, E.; Rothlisberger, U.; Keiser, J.; Gasser, G. Multidisciplinary Preclinical Investigations on Three Oxamniquine Analogues as Novel Treatment Options for Schistosomiasis. ChemRxiv. Preprint. 2020, DOI: 10.26434/chemrxiv.12102894.v1.
- Lin, Y.; Ong, Y. C.; Keller, S.; Karges, J.; Bouchene, R.; Manoury, E.; Blacque, O.; Müller, J.; Anghel, N.; Hemphill, A. et al. ChemRxiv. Preprint. 2020, DOI: 10.26434/chemrxiv.11982180.v1.
